|
|
|
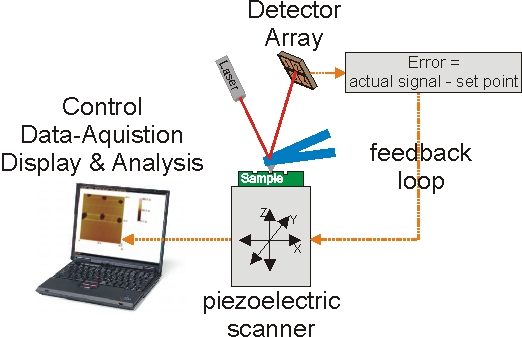
Scanning probe microscopy had its breakthrough with the development of the scanning-tunneling microscope (STM) by Heinrich Rohrer and Gerd Binning for which they received the Nobel Prize in Physics in 1986 1-3. Based on the principle of a piezoelectric scanning of a very sharp probe relative to a sample, several other methods were developed soon. They give access to chemical, topographical and optical information with high spatial resolution. Each of these technologies has its own characteristic powerful capabilities:
Our goal is an innovative, multidisciplinary approach for the investigation of cell communication at a molecular level by means of micro-fabricated, integrated scanning nano-probe and nano-sensing systems. An important component of cell communication is the exocytotic release of transmitters, their diffusion between cells and the selective binding to suitable receptors, which lead to the initiation of cellular signaling events specific to the transmitter and the receptor. Little is known about the formation of secretory granules and the characteristics of transmitter release, especially which can lead to functional changes that accompany a particular syndrome or disease. Many of these changes involve disruption of chemical communication between cells, most notably in the central nervous system, lungs, and kidneys. Hence, detailed knowledge and multi-parametric analytical assessment of exocytotic events will help understand the underlying mechanisms of cellular communication. The proposed multifunctional tool, based on a combination of scanning probe techniques will provide simultaneous information with a high selectivity for individual transmitters, high temporal resolution of changes in transmitter concentration, and high spatial resolution to distinguish which cells are releasing transmitter molecules, information required for in-situ investigations of complex biological systems and heterogeneous matrices. [1] G. Binning, et. al., Helv. Phys. Acta 55, 726-735 (1982). |
|
[Martin Brucherseifer] [About me] [Resume] [SEM / FIB] [Scanning Probe Microscopy] [THz Spectroscopy] [AIXScan] [Publications] [Links] [Disclaimer] |